- Q & A
- …
- Q & A


- Q & A
- …
- Q & A

Squirrel Medical®
Established in 2003, Squirrel Medical® is one of the UK’s only manufacturers exclusively focused on Class IIa active mattress and cushion systems, fully certified under UK MDR 2002.
Clinically Effective
Squirrel Medicals Active Mattresses systems are supported by outstanding independent clinical studies. Several of these have been presented at EPUAP and published at PUB MED. The entire clincal evaluation, a major body of work, has been audited by SGS. The study below, conducted by University of St Mark and St John, discovered that ones choice of mattress can significantly alter long term health.

Published Pub Med Results.
- Endothelial Function IMPROVED 197% (p=0.021)
- Resting Blood Flow IMPROVED 336% (p=0.003)
- Fat FREE Mass IMPROVED 520 grams (p=0.026)
- Thermoregulation at Feet IMPROVED 3.5ºC (p<0.001)
- Blood Pressure IMPROVED 120/77 to 115/75 (p=0.053)
- Participants reported a reduction in morning stiffness and joint pain.
- Research conducted by University of St Mark and St John, England.
(1) Baker G, Bloxham S, Laden J, Gush R. Vascular endothelial function is improved after active mattress use. J Wound Care. 2019 Oct 2;28(10):676-682. doi: 10.12968/jowc.2019.28.10.676. PMID: 31600104.
Regulatory Compliant
Squirrel Medical is proud to be among the few UK companies whose products meet the rigorous standards of UK MDR Class IIa compliance—a mark of safety, clinical evidence, and regulatory excellence. This classification isn't just a legal requirement—it’s a promise of protection. It ensures our medical devices have undergone detailed technical scrutiny, clinical evaluation, and ongoing post-market surveillance.
For care providers, this means reliable equipment backed by robust scientific validation. For patients, it offers the reassurance of proven safety, performance, and risk mitigation—critical in the prevention of avoidable harm such as pressure ulcers. When you choose Squirrel Medical, you're choosing a partner committed to regulated excellence and patient-centred outcomes.

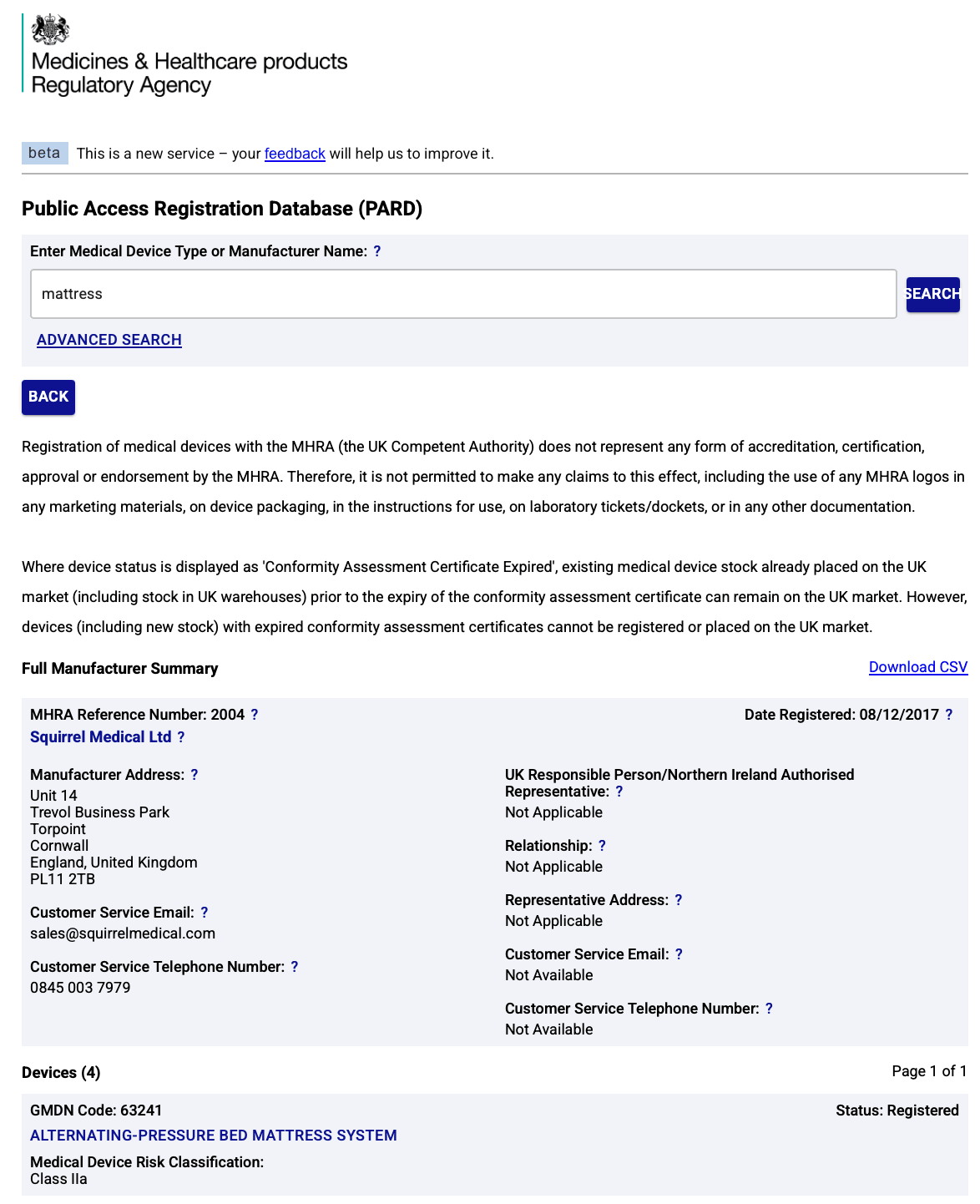
Prove Our Class IIa Status – Click to Verify
- Squirrel Medical’s active mattress systems are officially registered as UK MDR Class IIa medical devices.
👉 Click here to view our registration on the MHRA database
Regulated. Verified. Transparent.
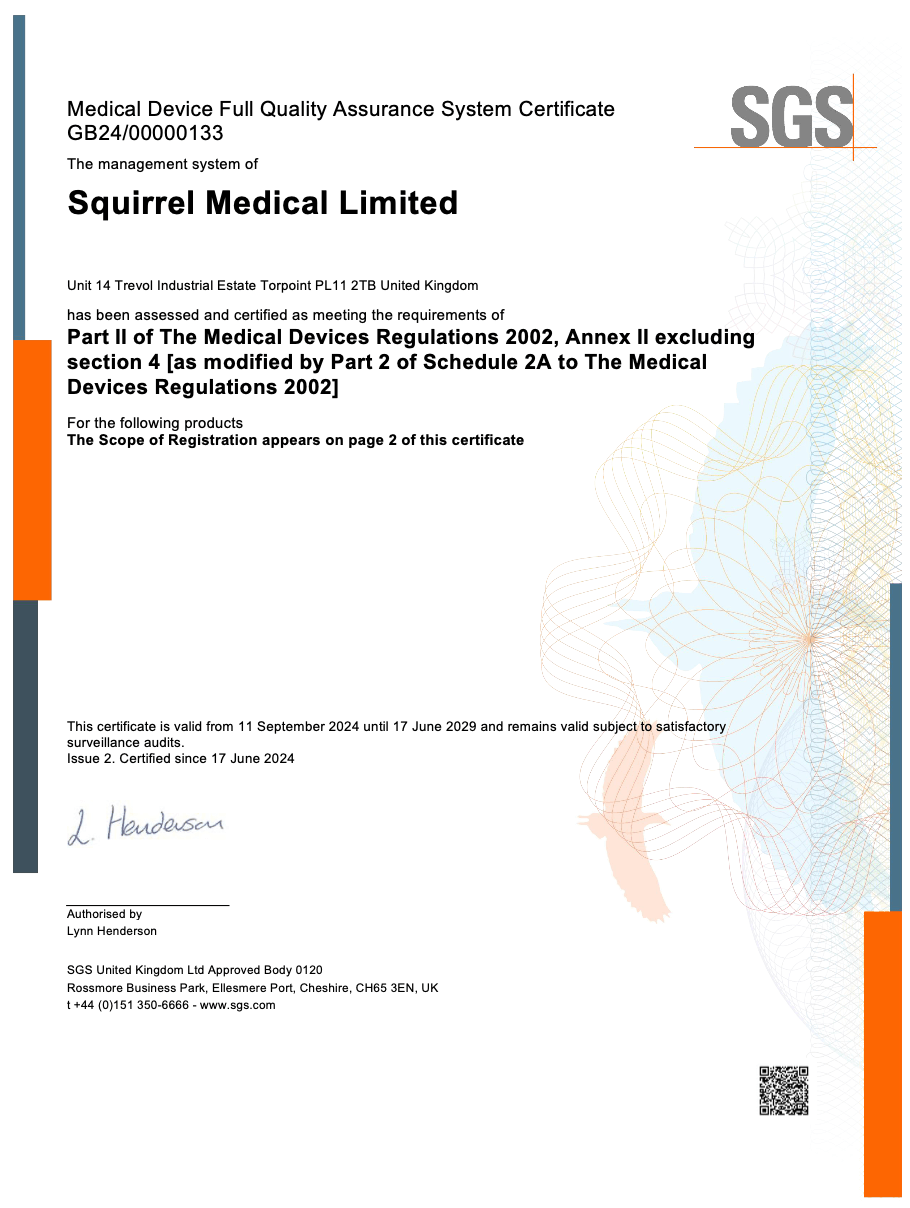
Certified by an Independent UK Approved Body
Squirrel Medical devices meet UK MDR Class IIa requirements and have undergone mandatory third-party conformity assessment by SGS UK (Approved Body 0120).
- Assessed under Part II of the Medical Devices Regulations 2002, Annex II
- Certified for active mattresses and seat cushions including Diamond, Vestims, Dormir, and Aurora
- Verified through full quality assurance system certification valid until 2029
👉 Click here to view our SGS-issued certificate
Legally compliant. Independently approved. Market ready.
Outstanding Mattresses. Trusted Everywhere.
At Squirrel Medical, our active mattresses are more than just support surfaces—they’re legally compliant, clinically effective, and protected by patent. Designed for durability and audited for quality, each system is safe, robust, and fully approved for use across care settings.
We offer four outstanding VeSTIMs® systems:
🏠 Two 15cm models – Ideal for home care and care homes, fully compliant with bedrail height regulations
🏥 Two 18cm models – Engineered for hospitals and acute care, compatible with all standard hospital beds
Whether in the community or the clinic, Squirrel Medical delivers compliant, high-performing solutions that adapt to real-world care needs.

Vestims Reactive 18
18cm deep cell design with egress alert.
UK MDR Class IIa

Vestims Advance 18
18cm deep cell fully automatic.
UK MDR Class IIa

Vestims Pressureof 15
15cm low profile active mattress, egress alert.
UK MDR Class IIa

Vestims Auto 15
15cm low profile Fully automatic.
UK MDR Class IIa
Trusted. Tested. Transparent.
Squirrel Medical is one of the UK’s few Class IIa-certified medical device manufacturers with products officially listed on the MHRA Public Access Registration Database and backed by third-party conformity assessment through SGS UK (Approved Body 0120). Our innovations are not only scientifically proven—with results published in PubMed—but also publicly verifiable and legally compliant. In every partnership, we bring together regulatory rigour, clinical credibility, and technological innovation to protect patients and support care teams with complete confidence.
We offer:
Fully compliant solutions backed by SGS certification and registered with MHRA.
Clinically proven safety and performance data
Flexible partnerships including OEM, dual-branding, and custom integrations
A strong focus on risk reduction and patient safety
Backed by independent trials and partnerships with leading care providers, our technology is proven in practice and protected by compliance—giving both caregivers and commissioners the confidence they need.
🔹 Setting the standard in compliant, evidence-based pressure care.
Contact Squirrel Medical
Call: 0845 003 7979
Mission Statement
At Squirrel Medical, our mission is to enhance patient comfort and safety by delivering innovative, clinically effective solutions for the prevention and management of pressure ulcers. We are committed to supporting healthcare professionals with high-quality, reliable equipment designed to improve outcomes in hospitals, care homes, and community settings. Through continuous research, responsive service, and a focus on dignity in care, we strive to be a trusted partner in pressure area management.
Copyright 2025 Squirrel Medical®
Caution: Squirrel Medical Active Mattresse's are subject to multiple patents, patents applied for and intellectual property.
Errors and Ommissions Excepted.