- Q & A
- …
- Q & A


- Q & A
- …
- Q & A

Regulatory Compliant
Active Mattress Systems
Fully certified under UK MDR 2002
Class 2a
Regulatory Compliance
When regulation is done right, it doesn’t hold innovation back — it moves it forward. The healthcare industry is entering a new era where transparency, traceability, and patient safety are non-negotiable. For active mattresses used to prevent pressure ulcers, these new rules are an opportunity — not a burden. By meeting Class IIa medical device standards, suppliers and care providers can demonstrate clinical credibility, secure legal peace of mind, and deliver better outcomes for patients. At Squirrel Medical, we see compliance as a mark of quality — one that sets us apart, earns trust, and drives progress in every care environment we serve.

The Fiasco That Changed Everything
In 2010, Europe witnessed one of the most alarming medical device failures in history: the PIP breast implant scandal.
A French company had used industrial-grade silicone — meant for mattresses and upholstery — in breast implants, exposing over 100,000 women across 60 countries to toxic material. These implants were prone to rupture, causing pain, inflammation, and long-term illness.
But the real tragedy?
💔 No traceability.
💔 No records.
💔 No accountability.
Thousands of women were never contacted, never warned, and never helped.The lesson was painfully clear:
When medical devices aren't traceable, lives are unprotected.

Obligations for Manufacturers & Distributors
At Squirrel Medical, we uphold the highest standards of regulatory integrity:
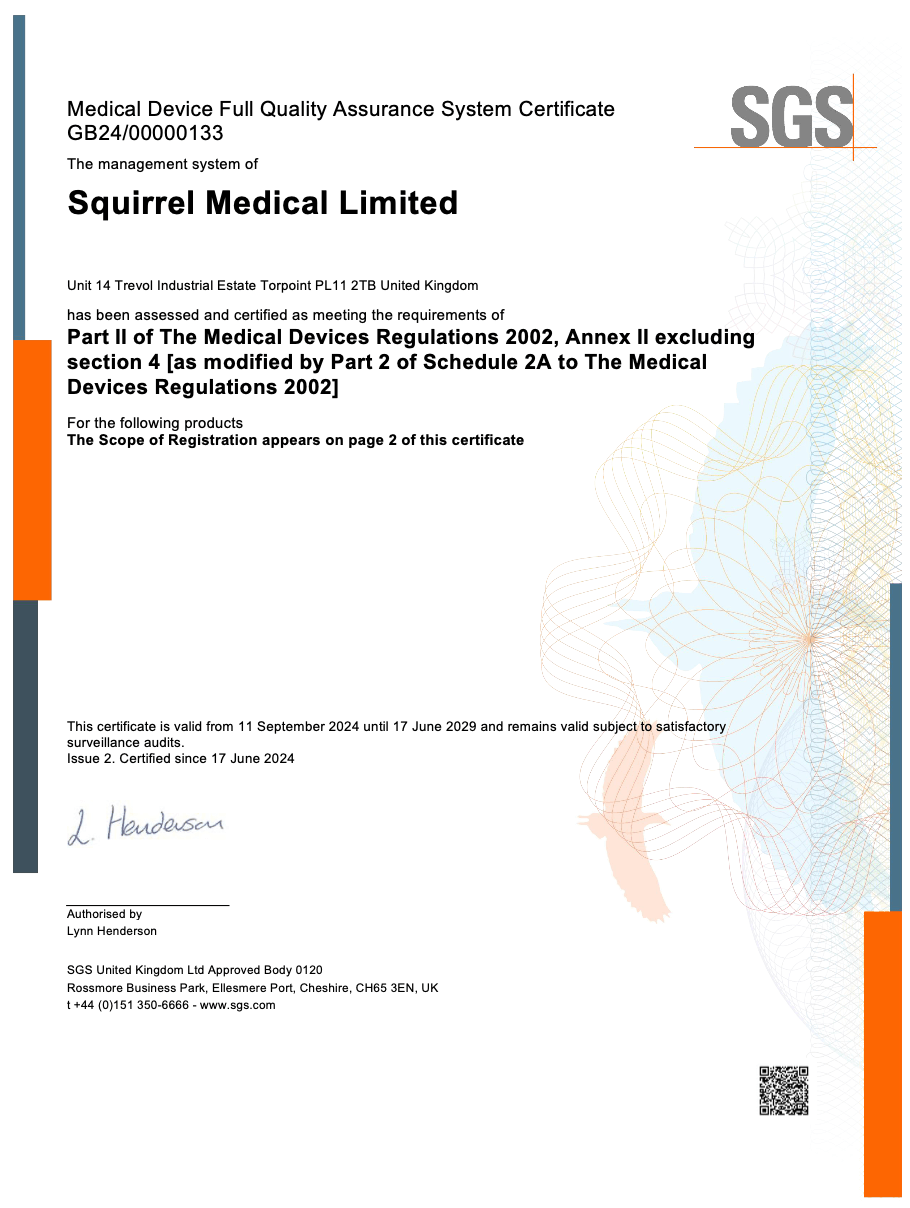
- ✅ Certified to ISO 13485:2016 (medical device QMS)
- ✅ Independently audited by SGS, an MHRA-approved Notified Body
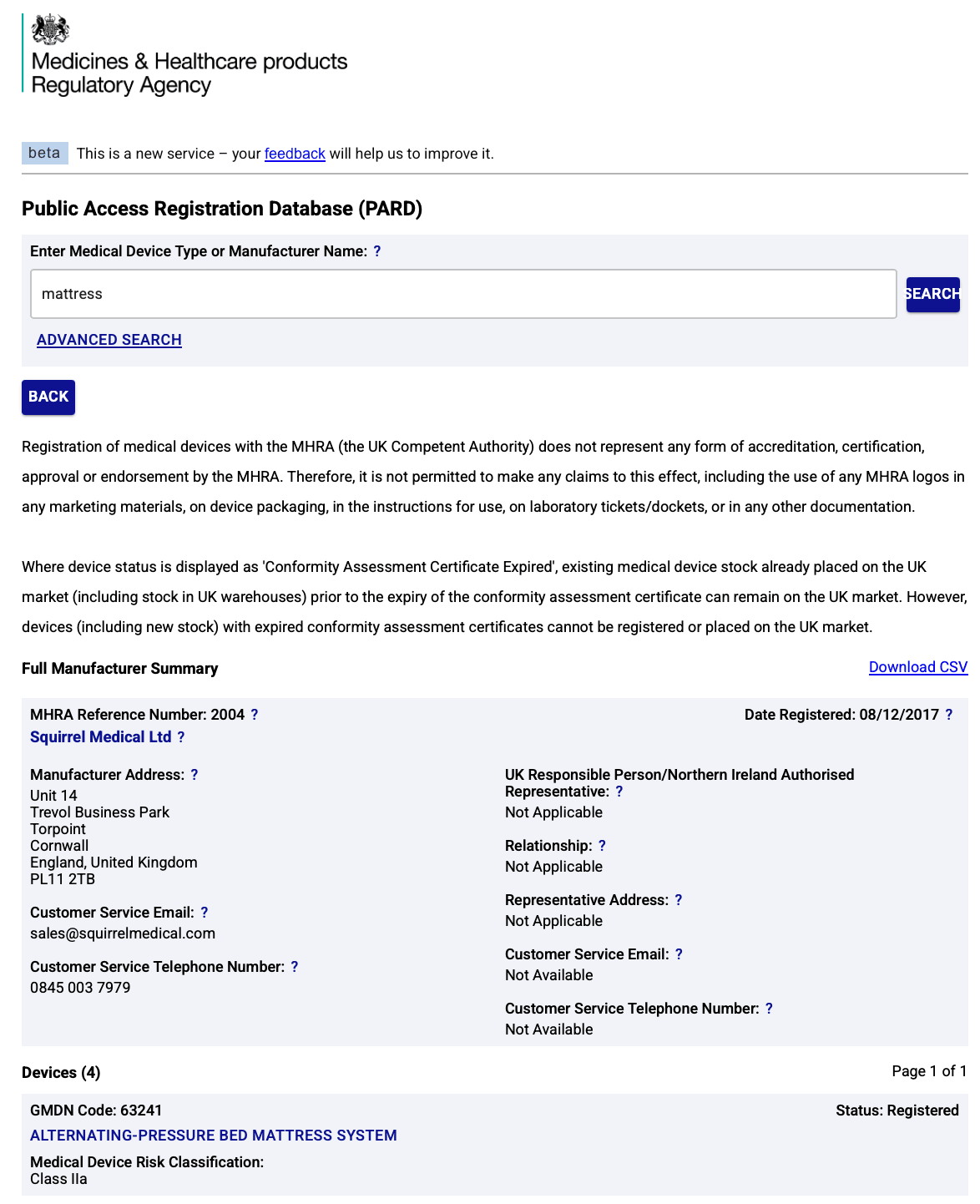
- ✅ Registered on the MHRA PARD database
- ✅ Product labelled with valid UKCA/CE mark and 4-digit Notified Body ID
- ✅ Backed by clinical data, traceability, and recall protocols
“If a person breaches enforcement under UK MDR, they may face imprisonment or fines.” — MHRA Regulation 60

Why Partner with Squirrel Medical?
We’re not just compliant — we’re committed.
- ✔️ Fully MHRA-registered (Class IIa)
- ✔️ Independently audited by SGS
- ✔️ Backed by peer-reviewed clinical data
- ✔️ Transparent documentation & recall-ready
- ✔️ Trusted by care homes, hospitals, and community providers
- “In this new regulatory landscape, compliance isn’t a bonus — it’s your shield.”
Copyright 2025 Squirrel Medical®
Caution: Squirrel Medical Active Mattresse's are subject to multiple patents, patents applied for and intellectual property.
Errors and Ommissions Excepted.